Transform Your Classroom into a Forensic Lab
Looking for a way to engage your students in hands-on science learning with real-world applications? Transform your classroom into a forensic science lab where students investigate fictitious crimes—and in the process, learn about physical and chemical properties, intermolecular forces, spectroscopy, and other key scientific concepts.
Go Direct SpectroVis® Plus Spectrophotometer
Introduce Your Students to Spectroscopy
Capable of connecting wirelessly or by USB, the
Go Direct SpectroVis Plus Spectrophotometer can easily collect a full wavelength spectrum (absorbance, percent transmittance, or intensity) in less than one second. Once the peak wavelength is determined, you can establish the concentration of a solution (Beer’s law) or monitor rates of reactions. A low light path allows this device to be used for microscale labs and biochemistry applications with micro and semi-micro cuvettes.
The Go Direct SpectroVis Plus Spectrophotometer employs an LED and tungsten bulb to transmit light through a high-quality diffraction grating. The diffracted light is sorted and collected by the linear CCD array detector.
GDX-SVISPL $479

Hands-on learning opportunities include
- Determine peak wavelength to collect data on solution concentration for studies of Beer’s law or to monitor rates of reaction.
- Collect a full wavelength spectrum to measure absorbance, percent transmittance, fluorescence (at 405 nm or 500 nm excitation), or emissions.
- Conduct enzyme kinetics experiments.
- Engage in equilibrium studies of absorbance vs. time or absorbance vs. concentration.
- Perform colorimetric or fluorescent bioassays. Use the Go Direct Fluorescence/UV-VIS Spectrophotometer for quantitative fluorescence analysis.
- Use the Spectrophotometer Optical Fiber to measure emissions from flame tests or other light sources. For more detailed emissions analysis, consider the Go Direct Emissions Spectrometer.
Hands-On Forensic Learning with the Go Direct SpectroVis Plus

The Case of the Poisoned Wine
Featured in the Forensic Chemistry Experiments lab book
Emergency services and police were dispatched to a private residence when party guests began to fall ill after drinking wine. Some were so ill they had to be transported to the hospital. Strangely, the effects on the guests varied; some were gravely ill while others were less so. Your job is threefold: determine the nature of the poison, determine the concentration of the poison in the contaminated wine, and finally explain why some guests were more severely affected than others.
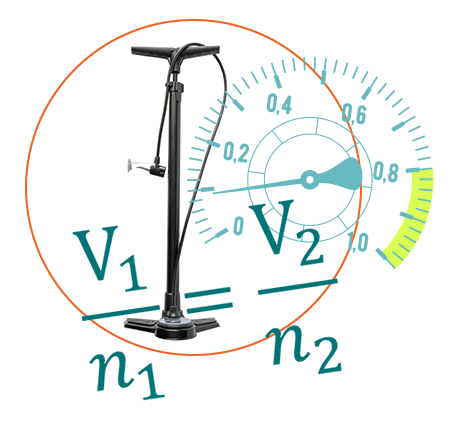
Introduce Your Students to Forensics with a Free Experiment
Get a preview of our new Forensic Chemistry Experiments lab book with one of our favorite forensic science experiments: “Avogadro’s Law and Order: Investigating a Rocket Launch Failure.” In this experiment, students investigate the details of a bottle rocket mishap to gain insights into the connection between gas pressure, the quantity of molecules within a confined space at a consistent temperature, and the significance of gas composition.
Teach students fundamental chemistry concepts through engaging forensic investigations. This lab book features 15 experiments specifically designed for introductory and advanced chemistry high school and college-level classes. Immerse students in the excitement of solving crimes through hands-on experimentation using Vernier data-collection technology! Teach gas pressure through a rocket launch failure, Beer’s law through a case of poisoned wine, and even more through engaging forensic investigations.
HSB-FCHEM-E $35
HSB-FCHEM $45

Hands-on learning opportunities include
- Using gas laws to determine how the relationships of pressure, volume, temperature, and number of molecules of a gas can help solve cases involved with gases
- Using absorbance, emission, and fluorescence spectroscopy to determine the contaminants in drinks and determine secret messages written with invisible inks
- Using conductivity and pH to compare the compounds in aqueous solutions for different water sources and mystery powders
- Applying the principles of thermochemistry and calorimetry to determine the heat of combustion of accelerants and the heat of solution of sweeteners
Go Direct pH Sensor
Monitor pH of Aqueous Solutions
The Go Direct pH Sensor is an important and versatile sensor for lab and field activities alike. It gives students the freedom to explore pH without the inconvenience of wires, and it transmits live readings and captures data in real time. This sensor consists of a pH electrode connected to a Go Direct Electrode Amplifier via a BNC connector. This design enables you to use the Go Direct Electrode Amplifier with other Vernier electrodes, such as the Go Direct Flat pH BNC Electrode, Go Direct ORP BNC Electrode, or ISE electrode, or a third-party electrode.
GDX-PH $109

Hands-on learning opportunities include
- Conduct acid-base titrations.
- Monitor pH change during chemical reactions.
- Test the pH and alkalinity of bodies of water.
- Investigate household acids and bases.
Go Direct Conductivity Probe
Determine the Ionic Content of an Aqueous Solution
The Go Direct Conductivity Probe determines the ionic content of an aqueous solution by measuring its electrical conductivity. It features a built-in temperature sensor to simultaneously read conductivity and temperature. Automatic temperature compensation allows students to calibrate the probe in the lab and then make measurements outdoors without temperature changes affecting data. This temperature compensation can be turned off to perform conductivity studies as a function of temperature.
GDX-CON $129
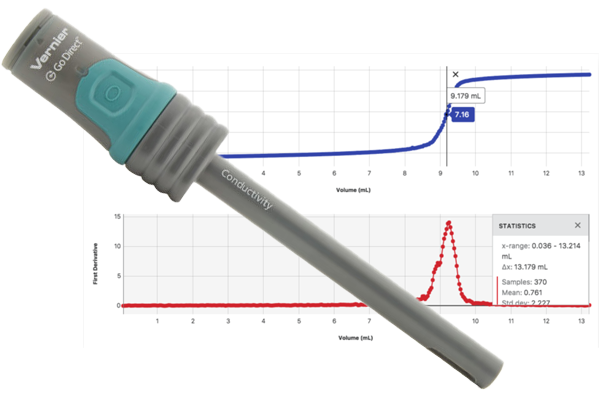
Hands-on learning opportunities include
- Demonstrate diffusion of ions through membranes.
- Investigate the difference between ionic and molecular compounds, strong and weak acids, or ionic compounds that yield different ratios of ions.
- Measure Total Dissolved Solids (TDS).
Hands-On Forensics Learning with the Go Direct pH Sensor and Go Direct Conductivity Probe

Mystery Powder from a Crime Scene
Featured in the Forensic Chemistry Experiments lab book
Police were called to the scene of a burglary where a wall safe was opened and jewelry was missing. On a table near the safe, a white powder was recovered by CSI technicians. Three suspects were identified who use or come into contact with white powder as part of their daily routine. Your job is to determine the nature of the white powder by determining some of its physical and chemical properties.
Go Direct® Colorimeter
Measure Absorbance or % Transmittance of a Liquid Sample
Use this sensor to explore absorbance and percent transmittance in a variety of experiments, including Beer’s law (absorbance vs. concentration) and kinetic studies (concentration vs. time). Students select among four wavelengths (430 nm, 470 nm, 565 nm, 635 nm) to set up their experiment.
The Go Direct Colorimeter features one-step calibration for all four wavelengths. It’s as simple as inserting a cuvette of distilled water and pressing the “Calibrate” button.
GDX-COL $135

Hands-on learning opportunities include
- Conduct Beer’s law investigations.
- Determine the concentration of unknown solutions.
- Study changes in concentration vs. time.
- Monitor reaction rates.
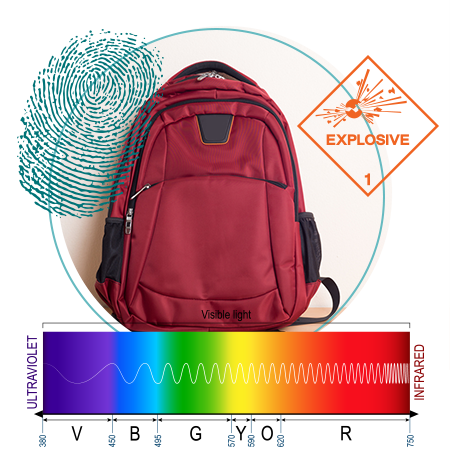
Color Countdown Timer
Featured in the Forensic Chemistry Experiments lab book
The bomb squad had been called to check a suspicious backpack left at the airport where they were able to diffuse the device. CSI techs also recovered fingerprints and a suspect has been apprehended. A note left in the backpack detailed how the bomb was supposed to detonate after a colored reaction proceeded to a certain point called the “half-life” of the reaction. Detectives would like you to testify in court and explain how you determined the half-life of the reaction and how this was supposed to detonate the device.
As you implement data-collection technology into your teaching, we’re here to support you! Looking to learn more about our forensics products or have questions about ordering? Reach out to our team at chemistry@vernier.com.
“My chemistry students really liked using Vernier sensors like pH, conductivity, and spectrophotometers when I recently tried out a new forensic toxicology lab. They got to work with real-time data and thought it was cool and fun to do the real-world tox screening.”
Diane Vrobel, EdD
Science Teacher at Archbishop Hoban High School, Ohio

